EMDN Codes: Hierarchical Structure and Application
Under the European Union's medical device regulations, MDR (EU 2017/745) and IVDR (EU 2017/746), it has become mandatory for all devices to be included in a common classification system. This classification system is defined as the European Medical Device Nomenclature (EMDN) and plays a critical role in the placing of devices on the market, traceability and technical file content.
What is EMDN?
EMDN (European Medical Device Nomenclature) is a free and open access device classification system developed by the European Commission and used in the EUDAMED database.
Derived from the Italian CND (CND) system. Launched on May 4, 2021, renewed in 2025. Free and open access, available to all producers across the EU are important features.
For all products covered by MDR and IVDR:
- EUDAMED records
- UDI-DI mapping
- Technical file product description
- It is mandatory to specify the EMDN code in the stages of notified body (NB) documents.
Hierarchical structure of EMDN
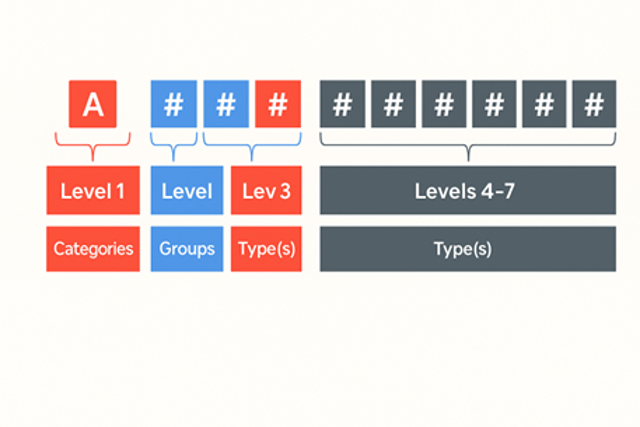
The EMDN uses a seven-level hierarchical structure similar to the Italian CND (Classificazione Nazionale Dispositivi medici) system. The levels are structured as follows:
- Category (Letter) – Broad device domain (e.g., A = Active devices)
- Group (Two digits) – More specific type within the category
- Type (Two digits) – Narrowed classification
- Unit (Two digits) – Specific grouping of products
- Level 5 – Descriptive grouping
- Level 6 – Detailed function
- Level 7 – Device-specific or unique identifiers (optional/coming later)
 Example:
Example:
- Code: A01AA01
- Interpretation:
- A: Active devices
- 01: Diagnostic imaging devices
- AA: Ultrasound imaging systems
- 01: General purpose ultrasound devices
This hierarchical breakdown allows for granular classification, which is vital for regulatory precision.
Application of EMDN codes
- EUDAMED registration
All manufacturers must assign the appropriate EMDN code to each of their medical devices when registering them in the EUDAMED database. This ensures uniform identification across the EU.
- UDI and traceability
EMDN codes support the Unique Device Identification (UDI) system by associating nomenclature with UDI records for traceability and post-market surveillance.
- Notified Body assessment
Notified bodies use EMDN codes to define their scopes of competence. Matching devices with notified bodies becomes more transparent.
- Procurement and reimbursement
Some member states use EMDN for health technology assessments, public tenders, and insurance reimbursement schemes.
Comparison with other systems (e.g., GMDN)
Though GMDN (Global Medical Device Nomenclature) has been widely used, EMDN is freely accessible and officially adopted by the EU. Key differences:
|
Feature |
EMDN |
GMDN |
|
Ownership |
European Commission |
GMDN Agency (UK) |
|
Access |
Free |
Subscription-based |
|
Regulation alignment |
EU MDR |
Global (non-MDR-specific) |
|
Language |
Multilingual |
English only |
Challenges and future outlook
- Mapping from legacy systems such as GMDN to EMDN requires effort and expertise.
- Updates and maintenance of the nomenclature depend on technological progress and regulatory needs.
- Artificial intelligence and automation tools may play a role in future EMDN code assignment.
Which sources should be considered when determining EMDN?
- MDCG 2021-12 Rev.1: EMDN user guide
- MDCG 2024-2 Rev.1: Update procedures
- MedTech Europe EMDN Guide (2025)
- EMDN browser: https://webgate.ec.europa.eu/dyna2/emdn/